Background
Measles virus in an enveloped single-stranded RNA virus with hemagglutinating and fusion glycoproteins.
1. If the patient needed to be admitted,
what isolation would be appropriate?
Negative-airflow respiratory isolation.
The virus is transmitted by inhalation via large droplet aerosols or by
airborne spread. The highest attack rates have been in childhood, usually
sparing infants less than 6 months of age because of passively acquired
antibody; however, a shift in age-specific attack rates to greater
involvement of adolescents and young adults was observed in the United
States in the 1980s. This shift is believed to be attributable to the
influence of immunization: younger children may be better immunized to limit
spread of the virus, whereas older age groups may have missed effective
immunization or earlier infection by the wild virus. A marked decline in
measles in the early 1990s may reflect decreased transmission as increased
immunization coverage takes effect. In the first half of 1993 only 167 cases
were reported by US health departments compared to 13,787 during the same
months of 1900, a 99% decrease.
Epidemics tend to occur during the winter
and spring in 1- to 3-year cycles and increasingly are limited to one dose
vaccine failures or groups who do not accept immunizations. The infection
rate among exposed susceptible subjects in a classroom or household setting
is estimated at 85%, and more than 95% of those infected become ill. The
period of communicability is estimated to be 3 to 5 days before appearance
of the rash to 4 days afterward.
2. What is known about the pathogenesis of
the infection?
After implantation in the upper respiratory tract, viral replication proceeds in the respiratory mucosal epithelium. The effect within individual respiratory cells is profound. Even though measles does not directly restrict host cell metabolism, susceptible cells are damaged or destroyed by virtue of the intense viral replicative activity and the promotion of cell fusion with formation of syncytia. This results in disruption of the cellular cytoskeleton, chromosomal disorganization, and the appearance of inclusion bodies within the nucleus and cytoplasm. Replication is followed by viremic and lymphatic dissemination throughout the host to distant sites, including lymphoid tissues, bone marrow, abdominal viscera, and skin. Virus can be demonstrated in the blood during the first week after illness onset, and viruria persists for up to 4 days after the appearance of rash. During the viremic phase, measles virus infects T and B lymphocytes, circulating monocytes and polymorphonuclear leukocytes without producing cytolysis. The effect of B lymphocytes has been shown to suppress immunoglobulin synthesis; in addition, generation of natural killer cell activity appears to be impaired. There is also evidence that the capability of polymorphonuclear leukocytes to generate oxygen radicals is diminished, perhaps directly by the virus or by activated suppressor T cells. This may further explain the enhanced susceptibility to bacterial superinfections. In addition, virion components can be detected in biopsy specimens of Koplik's spots and vascular endothelial cells in the areas of skin rash.

3. List the clinical signs that the physician focused on to make an accurate clinical diagnosis of measles. What test could be done to confirm the diagnosis?
Clinically, measles is usually so
characteristic that it is rarely necessary to perform laboratory tests to
make a diagnosis. Measles virus is difficult to isolate and grow. The virus
can be grown in primary human or monkey cell cultures. Respiratory tract
secretions, urine, blood, and brain tissue are recommended specimens.
Respiratory and blood specimens are best collected during the prodromal
stage up to 1 to 2 days after the appearance of the rash.
Antibody, especially IgM, can be detected
when the rash is present and is the best means to confirm the diagnosis.
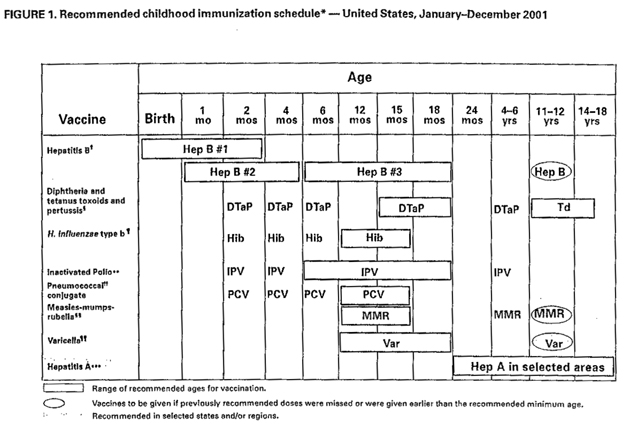
4. Describe the vaccine that is available
to prevent this infection. Why wasn=t this child vaccinated?
Vaccine coverage in many developing
countries (such as Mexico) is poor, and the child is at about the usual age
for vaccine.
A live attenuated measles vaccine in use
since 1963 has significantly reduced the incidence of measles in the United
States. The current Schwartz or Moraten attenuated strains of the original
Edmonston B vaccine are currently being used in the United States. Live
attenuated vaccine is given to all children after 12 months of age, in
combination with mumps and rubella vaccine (MMR vaccine). Although
successful results are greater than 95%, revaccination is being suggested
for children before grade school or junior high school. (A killed measles
vaccine, introduced in 1963 and subsequently discontinued, provided only
short-term immune protection. Recipients of killed vaccine were at risk for
the more serious atypical measles presentation upon infection).
Hospitals in areas experiencing endemic
measles may wish to vaccinate or check the immune status of their employees
to decrease the risk of nosocomial transmission.
5. Are there any effective anti-viral
agents that act against this virus? What is the appropriate treatment?
Should any therapy be prescribed for the 4 month old cousin?
Exposed susceptible individuals who are immunocompromised and <2 year old infants should be given immune serum globulin to modify their measles infection. This product is most effective if given within 6 days of exposure. [Babies <2 year old are at a higher risk for development of SSPE (subacute sclerosing panencephalitis)] There are no effective anti-viral agents. Treatment is supportive only.
6. Are there any long term consequences
associated with this viral infection?
Subacute sclerosing panencephalitis (SSPE)
is an extremely serious, very late neurological sequelae of measles that
occurs in about 7 in 1,000,000 patients. In SSPE a defective measles virus
persists in the brain and acts as a slow virus. The virus can replicate and
spread directly from cell to cell but is not released. Many months or years
after clinical measles the patient develops changes in personality,
behavior, and memory. Myoclonic jerks, blindness and spasticity follow.
Unusually high levels of measles antibodies are found in the blood and
spinal fluid. Eosinophilic inclusion bodies composed of paramyxovirus-like
nucleocapsids are present in the brains of patients with SSPE. The incidence
of SSPE has decreased markedly with the success of measles vaccination.
SSPE does not occur with immunization.