1. Should the patient be isolated?
No. HSV encephalitis is sporadic, not epidemic, and not contagious.
2. Was this likely an acute or recurrent
infection? Does HSV-2 cause encephalitis?
Encephalitis may rarely result from HSV-1
infection. Herpes encephalitis accounts for up to 10% of all cases of viral
encephalitis in the United States. Most cases occur in adults with high
levels of anti-HSV-1 antibody, suggesting reactivation of latent virus in
the trigeminal nerve root ganglion and extension of productive (lytic)
infection into the temporoparietal area of the brain. Primary HSV infection
with neurotropic spread of the virus from peripheral sites up the olfactory
bulb into the brain may also result in parenchymal brain infection.
HSV-2 can also cause encephalitis in
neonates, as a primary infection acquired from the mother.
3. What clinical findings suggest herpes
encephalitis as the diagnosis?
Classically, the disease affects one
temporal lobe, leading to focal neurologic signs and cerebral edema. If
untreated, mortality is 70%. Clinically, the disease can resemble brain
abscess, tumor, or intracerebral hemorrhage. In neonates, the infection is
less often focal.
4. Why was PCR used to detect this viral
infection instead of serology or virus isolation?
Rapid diagnosis is very important and
there are encouraging results suggesting that HSV can be detected in
cerebrospinal fluid by the polymerase chain reaction (PCR), although the
virus is rarely, if ever, cultured from this site. Brain biopsy with culture
of the virus from brain tissue has previously been the gold-standard method
of diagnosis.
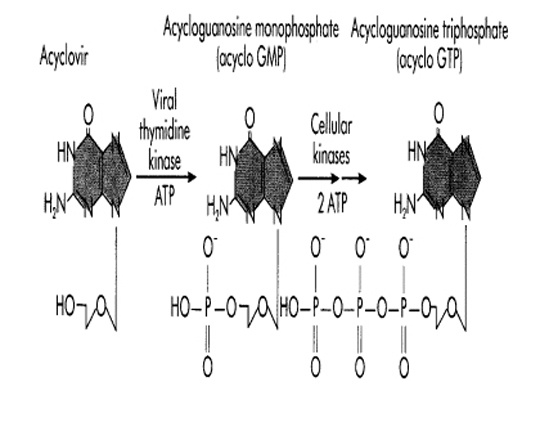
5. What is the mechanism of action of acyclovir?
Intravenous acyclovir is effective in inhibiting viral replication. Acyclovir (ACV) is a nucleoside analogue of guanosine. ACV has selective action against those herpesviruses that encode a Thymidine Kinase (TK). The viral Thymidine Kinase activated the drug by phosphorylation, and host cell enzymes complete the process to the triphosphate form. No initial phosphorylation occurs in uninfected cells, and thus there is no active drug to inhibit cellular DNA synthesis or to cause toxicity.

Activation of acyclovir (acycloguanosine)
in herpes simplex viral infected cells. Acyclovir is converted to
acycloguanosine monophosphate (acyclovir GMP) by the herpes-specific viral
thymidine kinase and then to acyclovir GTP by cellular kinases.
In herpesvirus infected cells, ACV
triphosphate competes with guanosine triphosphate and causes termination of the
growing viral DNA chain because there is no 3'-hydroxyl group on the ACV
molecule to allow chain elongation. The selectivity and minimal toxicity of ACV
is also due to its 100-fold greater utilization by the viral DNA polymerase than
by cellular DNA polymerase.
Mutations in either the herpesvirus TK or
polymerase can generate ACV resistant strains. These resistant strains are less
virulent but can still cause disease in immunocompromised patients.
Activity of ACV against herpesviruses directly
correlates with the capacity of the virus to produce TK. The order of TK
induction and ACV sensitivity is:
HSV-1 and >VZV>>EBV>>>CMV