Fragile X Syndrome
Fragile X syndrome occurs in individuals with a full mutation in the gene called FMR1, which is located on the long arm of the X chromosome (see Fig 1). The gene was named for “Fragile X Mental Retardation-1”. Fragile X syndrome is a trinucleotide repeat disorder. FMR1 alleles are broken down into categories based on the number of CGG repeats in the FMR1 gene (note that the repeat numbers are only approximates):
- Normal: ~5-40 repeats. These alleles are stable in size, and are transmitted to children without an increase or decrease in CGG repeat number.
- Intermediate alleles (or “gray zone”): ~41-58 repeats.
- “Premutation” alleles: ~59-200 or 230 repeats. These are not associated with mental retardation (MR), but women with alleles in this range are at risk for having a child with Fragile X syndrome since the allele can expand.
Full mutation alleles: Over 200 or 230 repeats. Several hundred to several thousand repeats is typical. Abnormal methylation of the FMR1 gene generally occurs. The full mutation causes moderate MR in affected males and mild MR in affected females.
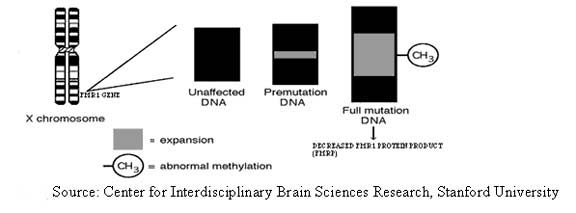
Figure 1.
Fragile X syndrome and the other disorders related to FMR1 (as will be discussed below) are inherited in an X-linked dominant fashion. Females who are premutation carriers have a 50% risk of transmitting an abnormal (premutation or full mutation) allele in each pregnancy.
The prevalence of Fragile X syndrome in males is estimated to be between 16 and 25 per 100,000. The prevalence of females affected with Fragile X is presumed to be about half the prevalence in males. The prevalence of females who have a premutation is thought to be quite high. One study showed a prevalence of 1/259; another showed a prevalence of 1/113. Clinical testing is available for both increased trinucleotide repeats and methylation changes in FMR1. Less than 1% of individuals with Fragile X syndrome have other mutations within FMR1, including deletions, point mutations or missense mutations. Prenatal testing is available for Fragile X syndrome.
Affected males (males with a full mutation) may have a characteristic appearance; many of these features become more apparent with age. They may have a large head, long face, prominent forehead and chin, protruding ears, joint laxity and large testes (after puberty). Behavioral abnormalities, sometimes including autism spectrum disorder, are common. There can be delayed attainment of motor milestones and speech, hyperactivity, hand flapping, hand biting, temper tantrums, tactile defensiveness, poor eye contact, perseverative speech, problems in impulse control, and distractibility.
Females with a full mutation allele can have the same physical and behavioral features seen in males, but with lower frequency and milder involvement. About 50% of females with a full mutation are mentally retarded, but are usually less severely affected than males with a full mutation. On the other hand, 50% of females with the full mutation are intellectually normal. The variability is thought to be due to X inactivation.
Two other conditions have been noted to occur in premutation carriers.
- The Fragile X-associated tremor/ataxia syndrome (FXTAS) occurs in some males with the Fragile X premutation. It is associated with late-onset progressive cerebellar ataxia and intention tremor. Penetrance is age related. Symptoms are seen in 17% of males ages 50-59, and up to 75% in males age 80 and older. Some female premutation carriers may also develop tremor and ataxia. It is estimated that 4.2% of men with adult-onset cerebellar ataxia, with no other affected family members, have a premutation in FMR1.
- FMR1-related premature ovarian failure (POF) occurs in about 20% of females that have an FMR1 premutation. The odds ratio for POF increases with increasing repeat size. Fragile X testing is therefore recommended in women with premature ovarian failure.
A 5 year old boy has been referred to see you. He has mental retardation, speech delay and autistic features. You note he has a long face and large ears. His chromosomes have already analyzed and the karyotype was normal (46,XY).
- What is your suspected diagnosis?
- Aside from mental retardation and autism, what other types of things would you ask about when taking a family history?
- What testing would you order?
- Your patient has an older and younger sister. Are they at risk? If so, for what, and what testing would you recommend or offer?